Bringing Technology to Market
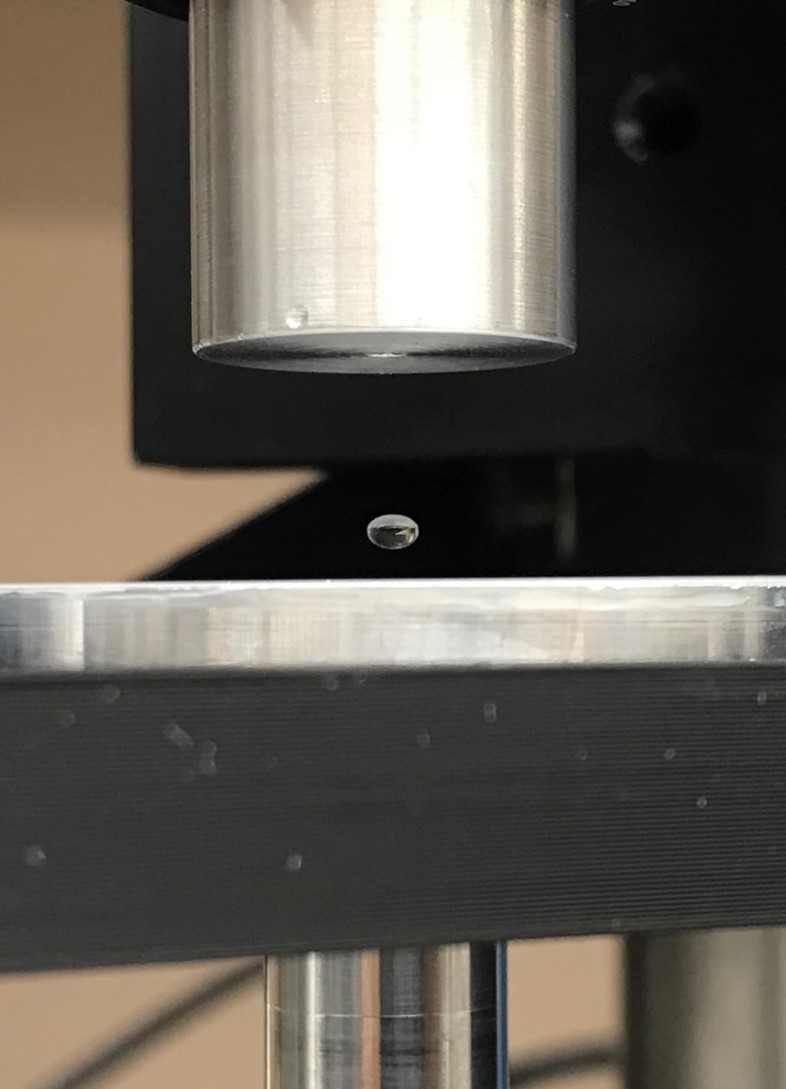
Monitoring blood coagulation is critical for neonatal and pediatric care, surgical patients, and for those on blood thinning medications. Current methods for measuring coagulation rates require 10 mL venipunctures of blood and expensive laboratory-based tests. These large blood volumes are a significant loss for sick patients and are almost impossible for children and infants to provide. Current blood coagulation tests are artificially triggered by contact with the instrument. To solve this problem, Levisonics, with NSF support, has developed a novel, non-contact blood coagulation test that uses acoustic force to levitate blood drops. To help bring the test to market, Fraunhofer USA CMI helped develop key components and automate drop deployment.
For the Levisonics test, just 6 μL of blood is required – a substantial reduction from current methods. Importantly, these blood volumes are available from simple finger or heel sticks, enabling widespread use in pediatrics and home care. In this platform, a drop of blood is levitated in an acoustic standing wave. Increasing the acoustic force gently squeezes the droplet and the degree of deformation is recorded on camera. As the blood coagulates and becomes more viscous, more force is required to deform the drop. This data is converted into a coagulation curve and has been shown to match the current gold standard. Fraunhofer USA CMI is automating the process such that the user simply inserts the blood sample and the liquid handling system automatically deploys the drop into the acoustic field for testing. This will allow blood coagulation measurements at the bedside, in local clinics, and ultimately, at home. Overall, the Levisonics platform is projected to improve the diagnostic response time up to 350% and decrease the cost of blood coagulation testing by over 30%. Projected savings for U.S. patients is at $2 billion annually.